CUSTOM RNAPOLS EVALUATION: OPTIMIZE THE PRODUCTION OF YOUR mRNA
Primrose Bio’s custom Prima RNApols evaluation program is advancing the field of mRNA therapeutics and vaccines by providing access to highly performing RNA polymerases that improve quality, efficiency, reduce costs, and enhance your mRNA production.

What are Prima RNApols?
Why Choose Prima RNApols
Evaluation Program Details

Power of Panel Case Study

What are Prima RNApols?
Prima RNApols are a proprietary collection of novel, improved and versatile RNA polymerases that produce mRNA products by in vitro transcription (IVT) of a DNA template.


Why Choose Prima RNApols
Primrose Bio has developed a panel of 20+ custom RNApols engineered to enhance IVT efficiency and outperform conventional T7 RNA polymerase in mRNA quality, yield, and purity.

Increased Yield
Increasing your overall yield and the efficiency in utilization of key reagents delivers on your mRNA manufacturing cost targets.

Reduction in dsRNA
Up to 100x reduction in double-stranded RNA improves the quality and safety profile of your mRNA.

Superior Quality
Higher integrity of target mRNA delivers both higher quality and lower processing costs.

High Capping Efficiency
Enabling both more efficient capping and better cap analog utilization delivers on cost, quality and efficacy targets.

Compatible with Modified Nucleotides
The ability to incorporate both natural and modified nucleotides provides you with the flexibility you need in drug design.

Drug Enabling
The flexibility in accommodating even the most challenging DNA templates opens new frontiers for mRNA therapies.

Evaluation Program Details
Screening (Weeks 1-3):
We identify 20+ enzymes tailored to your target mRNA substance and KPIs, such as yield, dsRNA content, integrity, and capping efficiency.
Process Optimization (Weeks 4-7):
We fine-tune IVT conditions and transfer optimized protocols for seamless integration into your applications.
Comprehensive Analytics (Weeks 6-8):
We perform best-in-class enzyme and mRNA analytics to ensure precision and quality.
Selection (Week 9-10):
Best-performing RNA polymerases are then shipped to you for further analysis, along with the resulting mRNA products.
TIMELINE
6-10 WEEKS
COST
STARTING AT $20,000

Power of Panel Case Study
Why rely on the power of the Prima RNApols Panel?
The following chart reflects a set of IVT experiments conducted with five Prima RNApols using defined process conditions and on a DNA template widely used for IVT process development.
The results highlight the variation in IVT performance between enzymes. Such variation increases rapidly using DNA templates of different lengths and sequences and/or when changing other reagents and process conditions. Screening the full panel for your specific materials, IVT process conditions and needs is the best way to find an RNApol that works for you.
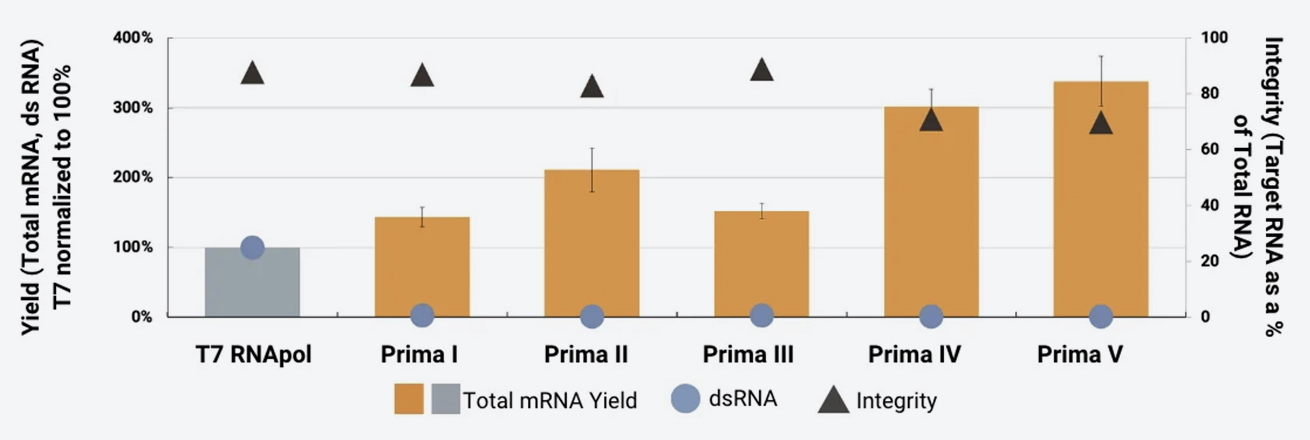
Total mRNA yield and integrity were measured on an Agilent Fragment Analyzer capillary electrophoresis system across all RNA sizes. The target yield was determined by quantifying the 5kb-sized peak. Integrity was calculated as the amount of the target RNA species as a percentage of total RNA. dsRNA levels were measured for equivalent amounts of total RNA synthesized in IVT reactions by immunoblotting with the J2 monoclonal antibody and quantitating spot intensities with ImageJ software.
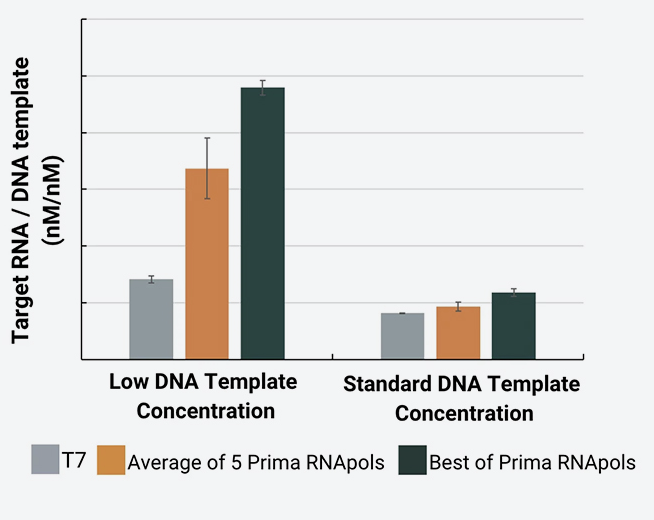
Total mRNA yield was measured on an Agilent Fragment Analyzer capillary electrophoresis system across all RNA sizes. The target yield was determined by quantitating the 5kb-sized peak.
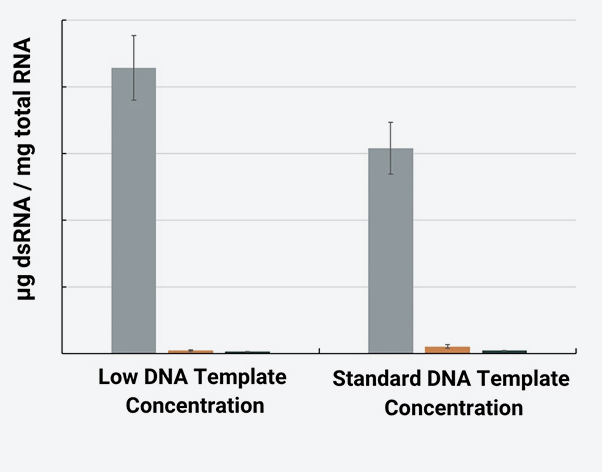
dsRNA levels were measured for equivalent amounts of total RNA synthesized in IVT reactions by ELISA using the SCICONSTM dsRNA ELISA Kit (based on the J2 monoclonal antibody).
